Alessandro Volta The Voltaic Pile Experiment The Invention of the Electric Battery
The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit. It was invented by Italian chemist Alessandro Volta, who published his experiments in 1799.

Reproduction of Voltaic Pile Science History Institute Digital Collections
Explore science From our collection Alessandro Volta's voltaic pile Alessandro Volta's voltaic pile The first electrical battery, invented by Volta in 1800 in Genoa, Italy. This voltaic pile, made before 1813, was presented to Michael Faraday by Volta in 1814. Date: pre-1814 Place made: Milan, Italy Alternative name: Early battery

"Plating out." Química, Aula de química, Clase de química
To construct a series of voltaic cells powerful enough to do visible work. Volta used zinc and silver; we will use zinc and more easily available copper. Materials 1. A strip of aluminum foil 2. About five or six pieces of 1-inch square cardboard or card stock saturated with sodium chloride solution 3. About five or six pennies (copper shell) 4.

Voltaic pile invented by the italian physicist Alessandro Volta (17451827). Colored engraving
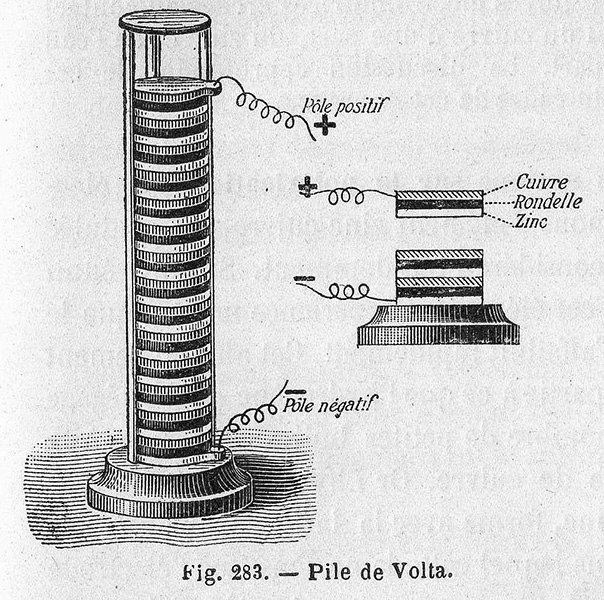
Operation. The first voltaic pile, invented by Alessandro Volta in 1800, was the first ever electrical battery. He piled up alternating copper discs and brine soaked fabric. When the end contacts were connected, a current flowed. This practical involves recreating the voltaic pile using copper coins and filter paper soaked in salt water.
Voltaic Pile ClipArt ETC
The finding led him to experiment with replacing the oil in lamps with methane and to create the Volta lamplighter. He rejects animal electricity. During those years, the inventor also travelled through Europe and came into contact with renowned intellectuals of the time, such as Horace-Bénédict de Saussure and Voltaire.. The voltaic pile.

Experiment Make a Voltaic Pile News about Energy Storage, Batteries, Climate Change and the
The Voltaic Pile and its Consequences. The next great step forward can be attributed to Alessandro Volta, who in 1799, following close upon the discovery of the galvanic effect by Galvani, built the first electric battery. This immediately became known as Volta's Pile and, like many other batteries which followed it, employed copper as an.
Voltaic Pile
This is also called a voltaic pile, which is named after Alessandro Volta, who created the first battery in 1800 by alternating zinc and copper electrodes with sulfuric acid between them. In Volta's battery and your penny battery, an oxidation reaction occurs at the zinc electrode that releases electrons and a reduction reaction occurs at the.

Voltaic pile by Newton & Co. Science Museum Group Collection
You can make your own voltaic pile out of simple and inexpensive materials. Although Volta used silver and zinc, it is more feasible - and inexpensive - to use copper and zinc for the metal disks.

Voltaic Pile In A Class Room Stock Photo Download Image Now Stack, Electricity, Laboratory
Voltaic piles quickly began showing up in laboratories and facilitated many scientific discoveries in the early 19th century. In a matter of months after Volta's device became public, William Nicholson and Anthony Carlisle used it to divide water into its basic components - hydrogen and oxygen.

Voltaic pile YouTube
Description: In 1800, Alessandro Volta of Italy announced his invention of a device that produced a small but steady electrical current. His "voltaic pile" operated by placing pieces of cloth soaked in salt water between pairs of zinc and copper discs, as seen in this 1805 pile from Canisius College.

Voltaic pile given to Faraday, 18131827, replica Science Museum Group Collection
Watch as Dr. Joel Bryan of Ball State University discusses the history of the voltaic pile and shows how you can make one yourself out of simple and inexpensive materials.

Original Voltaic pile. Probably made and used by Volta himself. This pile was shown at the
1 Introduction This is a story describing the challenge and entertainment of restaging the experiment to decompose water using electric energy from a voltaic pile in a history of science course.
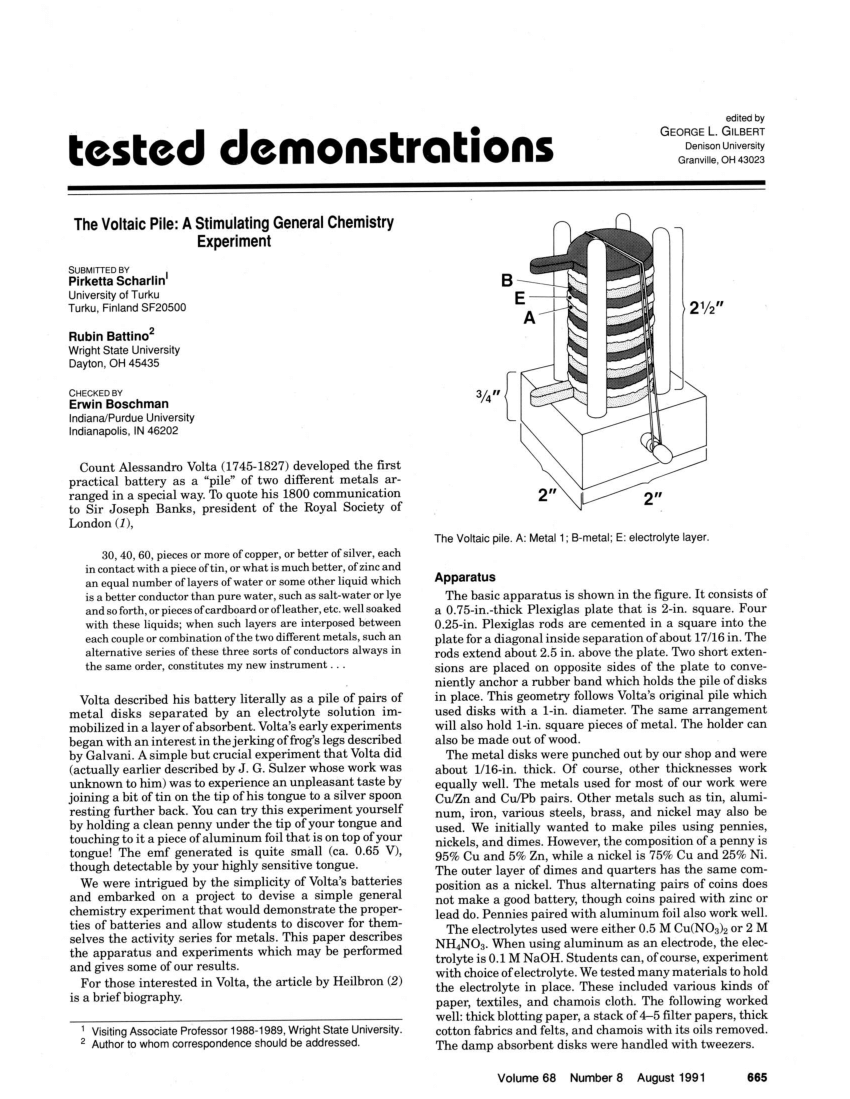
(PDF) The Voltaic pile A stimulating general chemistry experiment
The Voltaic Pail Experiment Basically, Volta's pile was a messy stack (pile) of discs made of two types of metal - one silver, the other zinc. The discs were separated from each other by a piece of cloth or cardboard that had been soaked in salt water (brine).

Making Voltaic Batteries in Mum's Kitchen News about Energy Storage, Batteries, Climate Change
Make a battery with pennies, nickels, salt, and vinegar in this fun science experiment! This type of battery is also called a voltaic pile. You can use a mul.

Pin auf Technology
A voltaic pile is an early form of electric battery. Italian physicist Alessandro Volta stacked piles of alternating metal copper and zinc discs separated by pieces of cloth or cardboard soaked in an electrolyte solution.
Battery Voltaic Pile National Museum of American History
Steps: Cut the aluminum foil and cardboard into circles. Set the cut outs aside. Make an acid mixture by mixing cider vinegar and salt in a saucer. Soak the cut out cardboard in the acid mixture. Tape the copper wire to one of the cut out aluminum foils. Alternately stack the aluminum foil, cardboard and coin.